ENVIRONMENTAL MONITORING
FOOD PRODUCTION LINES
SENSITIVES – PASTEURIZED – READY TO EAT
TECHNICAL SUPPORT AND TRAINING FOR IMPLEMENTATION
companies involved in these events, damages to the consumer who tries to educate themselves more and more in the healthiness of the product they consume, as well as regulatory pressures rise with events like this.
The manufacturer is obliged to produce safe food, where safe means harmless, which will not cause harm to the consumer at the time of consumption.
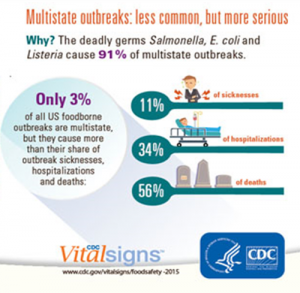
Listeria, Salmonella, E.coli become the vegetative pathogens that lead the statistics, because they are the microorganisms that generate the most impact at the time of multi-state outbreaks and that cause the highest mortality rate. Figure 1. shows that they are under multi-state outbreaks, however, when they occur they cause a lot of damage where 56% of consumers die and 34% are hospitalized.
SENSITIVE INGREDIENTS
The vulnerability of a food to become contaminated will be based on the form and type of ingredients with which it is composed, today the Law of Modernization of Food Safety – FSMA clearly establishes that there are ingredients with high vulnerability to be contaminated with microorganisms called “Sensitive Ingredients”, this leads to greater care at the time of transformation.
SANITARY DESIGN
Many times all efforts are devoted to the sanitary design of equipment, logically it must be so since that is where the product will have contact at the time of its manufacture. This does not become the only factor to take care for an adequate control, the sanitary design of the installation is a high priority, since they are the areas that normally come into contact with dirt from the same process. On the floors, walls and ceilings colonies of pathogenic microorganisms can be formed that could reach the product if they are not controlled under key programs as shown in Figure 2.
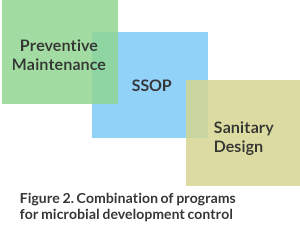
Figure 2. Combination of programs for microbial development control
READY TO EAT
The intention of use, this will add another factor of greater care, since there will not be a later step that will eliminate any presence of a microbial contaminant present the food, thus generating a condition of vulnerability to the consumer.
ENVIRONMENTAL MONITORING – MA
The food industry begins to improve its understanding regarding this program, many times as experts or auditors we ask the manufacturer if an MA program is carried out in their factory, many respond no and those who respond positively, assume that it is a methodology based on placing petri dishes exposed to the environment to determine possible microorganisms (fungi or aerobes). The important thing to understand about this program is that it gives more kindness than just knowing if we have fungi or aerobes present in the environment.
The food industry must avoid at all costs that the food is contaminated with pathogenic bacteria, such as Salmonella, Listeria or E. coli that arrive through different sources, Figure 3
- Operational practices
- Processed water
- SSOP failures
- Sanitary Design

Failure to interpret this program can lead to the manufacturer’s failure, it is essential to understand what the result of each of the data means.
While it is true that the purpose is to show that there is a presence of microorganisms, it is more important to interpret it through the following questions:

In order to answer these questions we must establish parameters or conditions that will help us systematize the MA program and result in the effectiveness we expect.
To achieve this, the first thing to know is:
- Type of food present
- Sentient
- Not sensitive
- Nature of waste
- Dry
- Damp
- Process type
- Open
- Closed circuit
ZONING
Segment the process area according to the degree of care that should be taken in each of them. Eg Raw process area differentiated from cooked process area.
Where we should pay more attention will be in the post-lethality area.
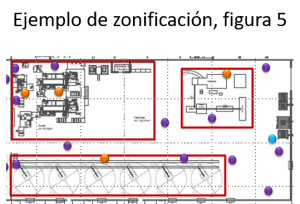
This will lead the manufacturer to a better interpretation because he knows which are the most critical areas of the process and the probability of contaminating the food.
FDA – FSMA
The Food and Drug Administration through FSMA establishes this program as a must through its CGMP.
Where it stipulates that the plant must have 4 types of Zones, within the zoning. Where Zone 1 – Contact with Food and Zone 4 – Internal perimeters of the plant.
HISOPADO
By already establishing a sampling area, then adequate sampling should be arranged to cover most of the zones, this swab can be under an individual or composite sample as long as some result is not altered by confusion in the sampling.
RESULTS
The interpretation of appropriate results will lead to an effective and decisive solution, since the root cause analysis must answer all the questions of why or because the presence of microorganisms that do not necessarily have to be present in zone 1 to arrive To be critical.




